For the first time in the world, successful analysis of the fine structure of carbon material surfaces using dynamic nuclear polarization magnetic resonance - Contributing to the development and promotion of next-generation carbon materials−
Professor Hironori Kaji from Kyoto University's Institute for Chemical Research, Assistant Professor Katsuaki Suzuki, Professor Kazuma Goto from the Center for Nano Materials Technology at Japan Advanced Institute of Science and Technology (JAIST), Graduate student Eika Ando from the Graduate School of Natural Science at Okayama University, Associate Professor Takashi Kobe from the Academic Research Institute of Natural Science at Okayama University, and Research Professor Yuta Nishina from the Interdisciplinary Fusion Research Core collaborated to achieve a successful analysis of the fine surface structure of carbon materials using Dynamic Nuclear Polarization Nuclear Magnetic Resonance (DNP-NMR) technique. They succeeded in amplifying the signals of surface functional groups such as methyl groups and hydroxyl groups on carbon surfaces, which were previously considered impossible with the DNP-NMR method. They also successfully observed trace amounts of methyl groups and hydroxyl groups that have a significant impact on the properties of carbon materials. This achievement contributes to the control of surface structures of carbon materials and the development of carbon materials tailored for various applications, thereby promoting their utilization.
Graphene and thin film carbon materials have been attracting attention as one of the next-generation carbon materials, and numerous studies have been conducted regarding their applications. There are several methods for the fabrication of graphene and thin film carbon materials, including the chemical oxidation of graphite to obtain oxidized graphene. This oxidized graphene can serve as a catalyst support for metal nanoparticles and can be composite with polymers, carbon nanotubes, and other materials. Therefore, it is expected to have various applications in fields such as catalysis for chemical reactions, electrode catalysts for fuel cells, as well as in the field of biomaterials including drug delivery systems.
Such carbon materials have numerous defect structures on their surfaces, known to contain surface functional groups such as hydroxyl groups, carboxyl groups, epoxy groups, and methyl groups. The properties of carbon materials are known to be significantly influenced by the types and quantities of these surface functional groups. Therefore, it is crucial to understand and control the state of these surface functional groups in material development.
Conventionally, analysis methods such as X-ray photoelectron spectroscopy (XPS) and temperature-programmed desorption (TPD) have been used to analyze the surface functional groups of carbon materials. Although these methods offer good sensitivity, their accuracy has been a challenge. On the other hand, nuclear magnetic resonance spectroscopy (NMR), which was utilized in this study, allows for highly accurate analysis of the types of functional groups. However, traditional methods have had low detection sensitivity, calling for a high-precision and high-sensitivity analysis method for the surface structure of carbon materials.
In this study, we attempted to improve the sensitivity of analysis using NMR (Nuclear Magnetic Resonance) by employing a technique called Dynamic Nuclear Polarization (DNP). DNP has been receiving attention as a method to observe hydrogen (1H)and carbon (13C) atoms in molecules in solution with high sensitivity. NMR is an analytical method that utilizes the phenomenon of atomic nuclei in a magnetic field absorbing specific frequencies of electromagnetic waves (radio waves) to observe the state of target atoms. It is widely used for chemical compound identification and MRI examinations in hospitals.
DNP-NMR is a groundbreaking technique that amplifies the signal intensity in NMR by transferring magnetization from radical molecules used for signal enhancement, which are present in the sample, to the atomic nuclei, by simultaneously irradiating the sample with microwaves (MW). This can amplify the signal intensity in NMR by more than 200 times. However, carbon materials pose challenges in DNP-NMR because they absorb microwaves, hinder efficient magnetization transfer, and experience temperature increase due to microwave absorption, leading to a reduction in DNP effect. As a result, signal amplification using DNP-NMR has been considered impossible for carbon materials until now.
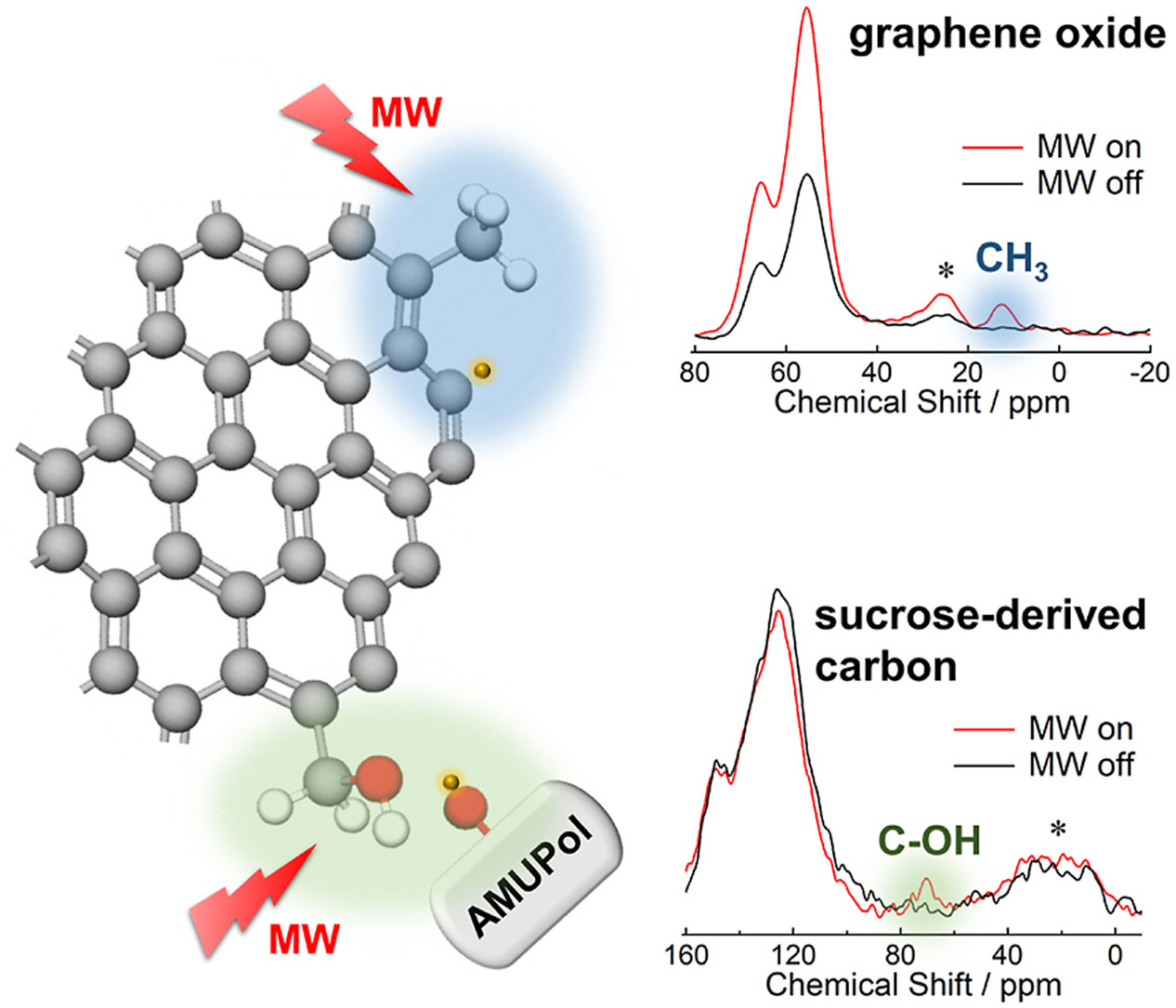
In this study, to enable signal amplification using DNP (Dynamic Nuclear Polarization), we changed the combination of the radical used for signal enhancement and the solvent from the conventional TEKPol/organic solvent system to the AMUPol/water system. This change allowed for the approach of radical molecules to the carbon surface, which is believed to have increased hydrophilicity due to the presence of hydroxyl groups and carboxyl groups, thereby realizing signal amplification using DNP. Additionally, we observed the phenomenon of signal amplification using DNP by utilizing intrinsic radicals present within the defect structures of carbon materials themselves.
Using this approach, we successfully observed methyl groups at the ends of oxidized graphene, which were hardly observed in conventional NMR measurements, by employing the 1H-13C CP/MAS solid-state NMR method. The signal amplification in this case was more than 10 times. Furthermore, we achieved signal amplification of more than 10 times for hydroxyl groups in amorphous carbon materials prepared by calcining sucrose.
This study is expected to advance the analysis of the fine surface structure of carbon materials using DNP-NMR in the future. By employing DNP-NMR and revealing the presence of trace amounts of surface functional groups remaining on the surface structure of carbon materials, it becomes possible to elucidate the differences in surface states of each carbon material. Consequently, it is expected to gain insights into the suitability of various catalyst elements for support. Determining compatibility can greatly contribute to the development of thin film carbon materials optimized for a wide range of applications in various fields.
●Terms Explanation●
support:It refers to the process of attaching materials such as metals to a substance that serves as a base for immobilizing other substances. Catalysts that support metals on graphene, for example, are industrially utilized as hydroxide catalysts and oxide catalysts.
NMR (Nuclear Magnetic Resonance): It is a spectroscopic technique that utilizes the phenomenon of "resonance" in which a sample placed in a magnetic field absorbs specific frequencies of electromagnetic waves. By observing these frequencies, it is possible to differentiate and detect functional groups such as hydroxyl groups, carboxyl groups, and methyl groups, making it widely used in the analysis of organic compounds.
DNP (Dynamic Nuclear Polarization):Dynamic Nuclear Polarization (DNP) is a technique that involves irradiating the sample with microwaves during NMR measurements to transfer the magnetization of nearby radicals to the target atomic nuclei. By significantly altering the difference in electron occupancy between energy levels during resonance signal detection in NMR, it is possible to obtain signal intensities several times or even more than 200 times higher than those in conventional NMR signals.
Radical:Atoms or molecules that possess unpaired electrons. They are highly unstable and reactive because they do not form shared electron pairs.
1H-13C CP/MAS solid-state NMR method: Cross-Polarization (CP) Magic Angle Spinning (MAS) NMR: An experimental technique where the magnetization of the 1H element is transferred to the 13C element under specific conditions. Additionally, the entire sample is rotated at a super-fast speed of several kHz or more, allowing for the high-sensitivity and high-precision detection of carbon NMR signals.
amorphous carbon materials: Unlike carbon materials with regular structures such as graphite, diamond, and carbon nanotubes, amorphous carbon refers to non-crystalline carbon that lacks a defined crystal structure. However, it does not mean that it lacks any organized structure; to some extent, it is known to have layered structures and internal pores. Hard carbon, a type of amorphous carbon, is used as the negative electrode material in lithium-ion batteries and sodium-ion batteries.

